Chemistry
July 6, 2021
The shape of nanoparticles in body fluids may help identify the type of cancer
A recent study by scientists from Japanese universities has shown that the shape of cell-derived nanoparticles, known as "extracellular vesicles" (EVs), in body fluids could be a biomarker for identifying types of cancer. In the study, the scientists successfully measured the shape distributions of EVs derived from liver, breast, and colorectal cancer cells, showing that the shape distributions differ from one another. The findings were recently published in the journal Analytical Chemistry.
Early detection of cancerous tumors in the body is essential for effective treatments. However, it is difficult to detect all types of tumors at an early stage, because detection methods differ among types of cancer, and some of the methods require painful medical procedures. It is thus vital to find methods that are painless and can detect multiple types of cancer.
Extracellular vesicles (EVs) are biological particles with a diameter of about 100 nanometers (nm) that are secreted from various cells and exist in body fluids like blood and urine. Notably, EVs contain biological molecules that carry information about the cells of the secretion sources. In addition, genetic materials contained in EVs are involved in communications among cells. This suggests that analyzing biological molecules in EVs present in body fluids could help to detect and identify some kinds of cancerous tumors in the body.
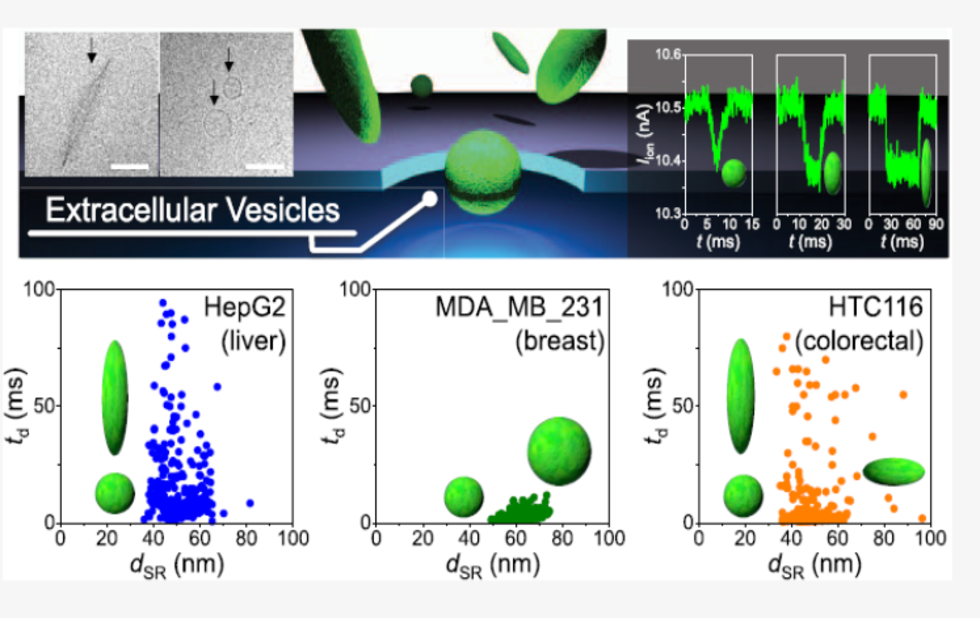
Previous studies had observed images of various shapes of EVs with electron microscopes. However, no technology existed to investigate the shapes of EVs distributed in body fluids, mainly because of difficulties in measuring the shape of nanomaterials in solution. In this context, a team of researchers from Kyushu University, Nagoya University, Osaka University, and Tokyo Medical University tried to investigate the shape of individual EVs in a fluid using a device that they had developed, which can analyze the shape of single particles in solution by measuring the change of ionic current flowing through pores of 200 nm in diameter when particles pass through there.
Using the device, the team successfully measured the shape of EVs derived from cultured liver, breast, and colon cancer cells, as well as from cultured normal breast cells, and found that their shape distributions differ from each other. For example, EVs derived from liver cancer cells include a mix of spherical particles and oval (like rugby balls) particles, whereas EVs from breast cancer cells consist solely of spherical particles.
Low-aspect-ratio nanopore devices can rapidly analyze the shapes of extracellular vesicles (EVs) individually in solution, and the present results reveal a dependence of EV shape distribution on the type of cells (cultured liver, breast, and colorectal cancer cells and cultured normal breast cells) secreting EVs. (Image Credit: Sou Ryuzaki)
The team then compared shape distributions of EVs in blood samples from breast cancer patients and non-cancerous individuals. The results showed that the shape distributions of EVs from the two groups differ from each other, indicating that measuring shape distributions of EVs in blood could distinguish patients with breast cancer from non-cancerous individuals.
"In this study, we found that measuring shape distributions of EVs in body fluids could identify the type of cancer," said Associate Professor Takao Yasui at the Nagoya University Graduate School of Engineering, one of the authors of the study.
The researchers note that measuring a greater variety of types of EVs will provide them with a more accurate idea of potential EV shape distributions as an index for cancer detection, screening, and diagnosis.
The paper, "Rapid Discrimination of Extracellular Vesicles by Shape Distribution Analysis," was published online in the journal Analytical Chemistry on April 28, 2021, at
https://pubs.acs.org/doi/10.1021/acs.analchem.1c00258
Authors:
Sou Ryuzaki, Takao Yasui*, Makusu Tsutsui, Kazumichi Yokota, Yuki Komoto, Piyawan Paisrisarn, Noritada Kaji, Daisuke Ito, Kaoru Tamada, Takahiro Ochiya, Masateru Taniguchi, Yoshinobu Baba*, and Tomoji Kawai
*Affiliation: Graduate School of Engineering at Nagoya University
Media Contact:
Takao Yasui
Associate Professor, Nagoya University Graduate School of Engineering
Email: yasui@chembio.nagoya-u.ac.jp
Naomi Inoue
Public Relations Office, General Affairs Division, Nagoya University
Email: kouho-en@adm.nagoya-u.ac.jp