Medicine, Dentistry, and Pharmacy
April 13, 2023
Alternative route used by specialized immune cells to colonize the embryonic brain suggests new approach to fighting fetal brain dysfunction
A team from the Nagoya University Graduate School of Medicine in Japan has uncovered new information about how microglia, the resident immune cells in the brain, colonize the brain during the embryonic stage of development. Although erythromyeloid progenitors (EMPs) were previously thought to divide into either microglia or macrophages, the group found that macrophages that enter the brain primordium —the brain in its earliest recognizable stage of development— can become microglia at later stages of development. These findings demonstrate the plasticity of these cells and suggest a novel approach to combat diseases affected by microglial imbalances, such as fetal brain dysfunction. Their study was published in Cell Reports.
In addition to neural lineage cells, the brain contains other types of cells, such as immune cells and vascular cells. These different cells work together to maintain optimal brain function. Among the immune cells of the brain, microglia are important for maintaining the health and function of the central nervous system.
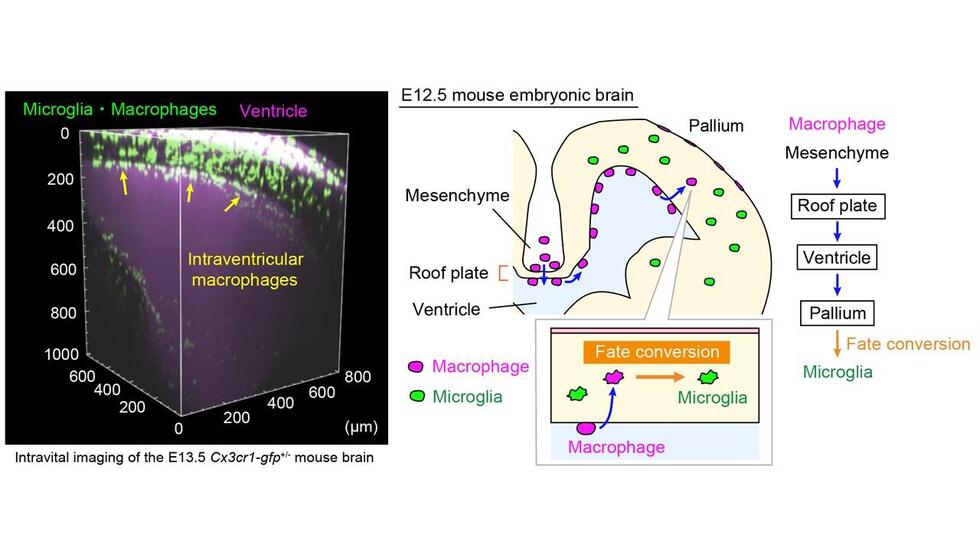
During embryonic development, EMPs differentiate into a variety of cell types, including macrophages and microglia. It was previously reported that segregation of EMPs into these two cell types usually occurs early in embryonic development in the yolk sac.
Using newly established intravital imaging system and cell-tracking methods, research by Lecturer Yuki Hattori (she/her) and Professor Takaki Miyata in the Department of Anatomy and Cell Biology; Professor Hiroaki Wake and Lecturer Daisuke Kato in the Department of Anatomy and Molecular Cell Biology; and Associate Professor Hiroyuki Konishi in the Department of Functional Anatomy and Neuroscience, Nagoya University Graduate School of Medicine, in collaboration with Okayama University, Kyushu University, and Freiburg University demonstrated that brain microglia are not only derived from yolk sac EMPs early in development but also supplied later during brain development by conversion from macrophages.
A type of macrophage called an intraventricular CD206+ macrophage, which is found along the inner surface of the cerebral wall, frequently enters a brain area called the pallium during the embryo’s development. Subsequently, these infiltrating macrophages differentiate into microglia in response to surrounding environmental factors in the brain. Dr. Hattori calls this the “additional route” in the brain that supplies some microglial populations.
To confirm their findings, the researchers transplanted macrophages into the brain’s ventricles. Surprisingly, intraventricular cells near the brain wall retained their macrophage properties, while those in the pallium acquired microglial characteristics.
“Our findings shed light on the possibility that differences in the routes of microglial colonization or timing of entry into the pallium part of the brain may be among the reasons for the diversity of microglial characteristics,” Hattori said. “There are at least several populations of microglial cells that can be distinguished by their routes of colonization into the brain. Although most microglia in the brain colonize the brain at a very early stage as microglia cells, about one-sixth of the microglia are derived later from macrophages.”
“Our findings offer potential solutions to problems such as fetal brain dysfunction,” she continues. “Recently, several studies have reported that increased maternal inflammation during pregnancy is associated with the onset of psychological brain defects in offspring later in development. Understanding the behavior of microglia in the physiological stage is important for studying their abnormalities in the inflammatory state and will contribute to the establishment of a unique preventive and therapeutic platform.”
The study, "CD206+ macrophages transventricularly infiltrate the early embryonic cerebral wall to differentiate into microglia," was published in the journal Cell Reports on February 7, 2023, at DOI: 10.1016/j.celrep.2023.112092
Authors:
Yuki Hattori, Daisuke Kato, Futoshi Murayama, Sota Koike, Hisa Asai, Ayato Yamasaki, Yu Naito, Ayano Kawaguchi, Hiroyuki Konishi, Marco Prinz, Takahiro Masuda, Hiroaki Wake, and Takaki Miyata
Media Contact:
Matthew Coslett
International Communications Office, Nagoya University
kouho-en@adm.nagoya-u.ac.jp